ACS Medicinal Chemistry Letters Synthesis and Biological Screening of Piranolquinoline Anti-inflammatory and Anticancer Agent Analogues

1. Article overview
Chronic inflammation is associated with many types of cancer, most of which solid tumors show signs of inflammation. Immune cells play an important role in the initiation, growth and progression of tumors. Some of these effects are regulated by pro-inflammatory cytokines, such as tumor necrosis factor (TNF)-α and interleukin 6 (IL-6). TNF-α and IL-6, as the main regulators of tumor-related inflammation and tumorigenesis, have become attractive targets for adjuvant cancer therapy. 1 In the context of treatment, quinoline analogs show diversified biological activities depending on the type of structure. As part of the number of natural products, 2,4-dihydroxyquinoline is an important compound and also exhibits various interesting pharmacological properties. Alkaloids containing pyridone and quinolone drug moieties show a wide range of biological activities. Many of these alkaloids, such as fungicides, quinolinone b, and exhibit cytotoxicity to cancer cells, are known as potential anticancer drugs.
Two, graphic guide
Chronic inflammation is associated with many types of cancer, most of which solid tumors show signs of inflammation. Immune cells play an important role in the initiation, growth and progression of tumors. Some of these effects are regulated by pro-inflammatory cytokines, such as tumor necrosis factor (TNF)-α and interleukin 6 (IL-6). TNF-α and IL-6, as the main regulators of tumor-related inflammation and tumorigenesis, have become attractive targets for adjuvant cancer therapy. 1 In the context of treatment, quinoline analogs show diversified biological activities depending on the type of structure. As part of the number of natural products, 2,4-dihydroxyquinoline is an important compound and also exhibits various interesting pharmacological properties. Alkaloids containing pyridone and quinolone drug moieties show a wide range of biological activities. Many of these alkaloids, such as fungicides, quinolinone b, and exhibit cytotoxicity to cancer cells, are known as potential anticancer drugs.
Two, graphic guide
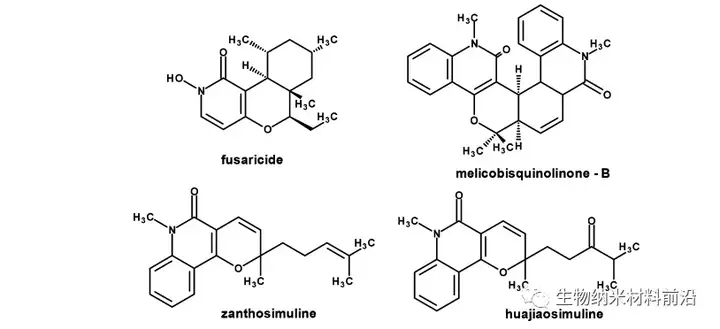
Figure 1. Pyridine and anticancer alkaloids based on quinolones.
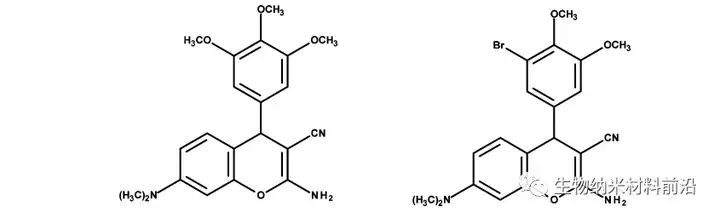
Figure 2. Color films are being studied as anti-cancer drugs.
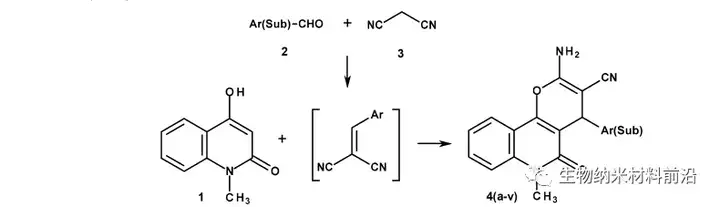
Figure 3. Synthesis reaction of 2-Amino-6-methyl-5-oxo-4-sub(aryl)-5,6-dihydro-4H-pyrano[3,2-c]quinoline-3-carbonitrile.
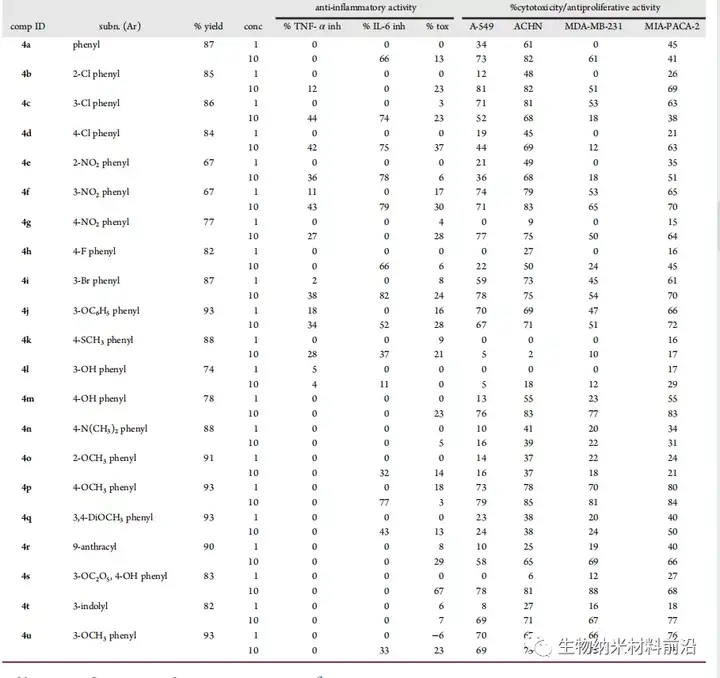
Figure 4. Anti-inflammatory and anti-proliferative activity data.

Figure 5. Anti-inflammatory and anti-cancer agent activity data.
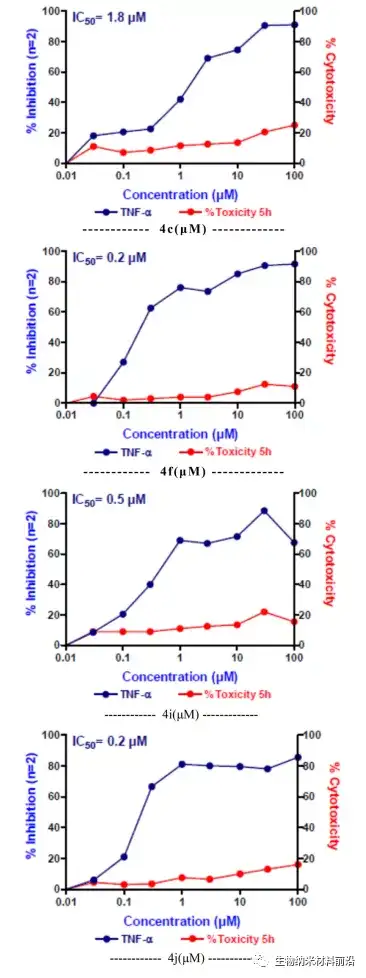
Figure 6. The effects of 4c, 4f, 4i, and 4j on the production of TNF-α by lps-stimulated cells in in vitro assays.
3. Full text summary
A series of new pyrano[3,2-c]quinolinine analogues were synthesized by multi-component reaction, and their anti-inflammatory and anti-cancer activities were evaluated. The screening results showed that compounds 4c, 4f, 4i and 4j are the candidates with the most anti-inflammatory and anti-cancer activities in this series. The structure-activity relationship is discussed, and it is shown that the 3-substituted [3,2-c]quinolone structural motif of the pyrano aryl ring seems to be an important position for TNF-α and IL-6 inhibition and anticancer activity. However, the structural diversity of electron-withdrawing, electron-donating, steric hindrance, and heteroaryl substitution sincerely affects inflammation and anticancer activity. The same preliminary research can identify a lead molecule that can be used in clinical trials.
Article link:
http://n.ustb.edu.cn/https/77726476706e69737468656265737421e0e2438f69316b4330079bab/doi/pdf/10.1021/acsmedchemlett.7b00545
3. Full text summary
A series of new pyrano[3,2-c]quinolinine analogues were synthesized by multi-component reaction, and their anti-inflammatory and anti-cancer activities were evaluated. The screening results showed that compounds 4c, 4f, 4i and 4j are the candidates with the most anti-inflammatory and anti-cancer activities in this series. The structure-activity relationship is discussed, and it is shown that the 3-substituted [3,2-c]quinolone structural motif of the pyrano aryl ring seems to be an important position for TNF-α and IL-6 inhibition and anticancer activity. However, the structural diversity of electron-withdrawing, electron-donating, steric hindrance, and heteroaryl substitution sincerely affects inflammation and anticancer activity. The same preliminary research can identify a lead molecule that can be used in clinical trials.
Article link:
http://n.ustb.edu.cn/https/77726476706e69737468656265737421e0e2438f69316b4330079bab/doi/pdf/10.1021/acsmedchemlett.7b00545
This information is sourced from the Internet for academic exchanges only. If there is any infringement, please contact us to delete it immediately.
18915694570
Previous: ACS NANO: Ti3C2Tx MXen


